Covalent’s Broad Platform Should Yield A Deep Catabody and E-Vaccine Pipeline

Basis for Platform Expansion
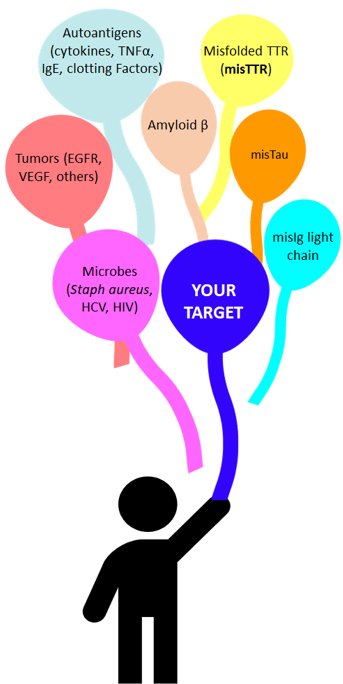
Covalent has gathered initial proof-of-concept for broad utility of the technology. In addition to our lead products, early stage catabodies and E-vaccines were identified for development against various microbial toxins, autoimmune disease targets and cancer targets (e.g., Staphylococcus aureus; HIV and Hepatitis C virus proteins; cancer-associated epidermal growth factor receptor), and autoantigens involved in autoimmune disease and allergy (VIP, immunoglobulins, coagulation factors, cytokines). We have two non-dilutive mechanisms for expanding the platform utility: contractual supply of catabodies/E-vaccines to Pharma companies for further commercial development, and (b) NIH R&D grants.
For example, Covalent’s discovery stage strengths can be readily focused on identifying lead catabodies directed to essential virulence factors without which drug-resistant Staph aureus is unable to establish infection. Likewise, electrophilic analogs of the virulence factors (E-vaccines) hold potential as the first effective vaccine against this intractable bacterium.
Longevity vaccination to prolong human life requires destruction of only the misfolded amyloid protein version. Off-target reactivity with the essential, properly folded protein form is not allowed. The foregoing objectives are feasible because Darwinian catabodies display exquisite specificity to misfolded proteins. From the precedents of the HIV E-vaccine, the electrophilic analogs of misfolded amyloid proteins have good potential as longevity vaccines that slow aging significantly.